Hox14
The enigmatic role of Hox14 in development and evolution
The Hox genes play central roles in the generation of animal body plans. Hox genes are arranged in clusters, and the position of each gene in the cluster coincides with where and when that gene is activated along the body. This correspondence between chromosome position and expression pattern is called ‘colinearity.’ In vertebrates, nested colinear Hox expression is found in the primary body axis, the nervous system, the muscular system, the gut, and the appendages. Studies in vertebrate genetic model species have dissected many aspects of Hox gene regulation and have demonstrated their critical function in patterning.
Interestingly, vertebrate genetic models have up to 13 members in each Hox cluster, but evolutionary analysis has revealed that ancestral vertebrate Hox cluster possessed a 14th Hox gene. While Hox14 paralogs have been lost repeatedly in model species, many groups retain Hox14 paralogs. The presence of Hox14 in these lineages correlates with unique morphological features, raising questions about the role of Hox14 in evolutionary transformations. In addition, assessing how the incorporation of a 14th Hox cluster member impacts aspects of Hox regulation such a colinearity and posterior prevalence are unknown, as are the transcriptional targets of Hox14.
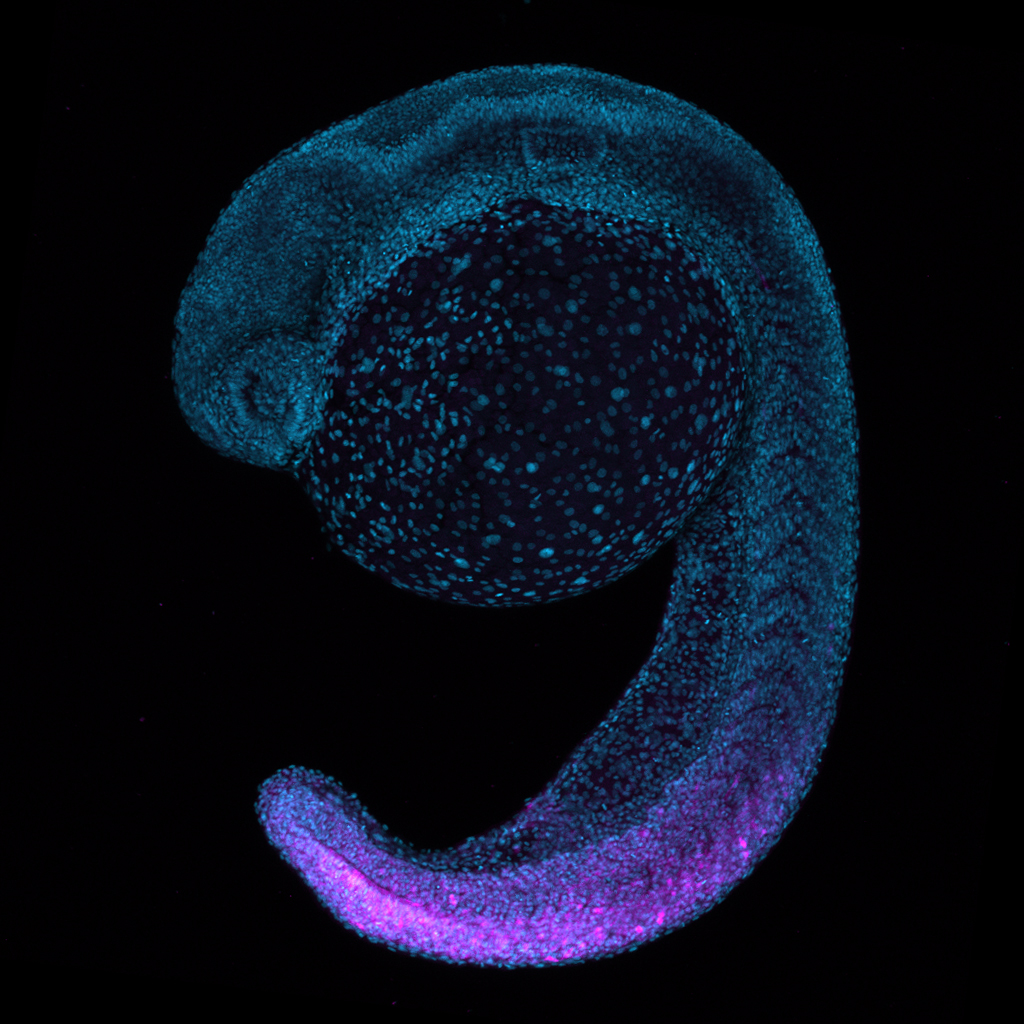
To interrogate the role of Hox14 in development and evolution, I am generating zebrafish tools that permit the study of Hox14 function. Like other vertebrate genetic models, zebrafish have lost all Hox14 paralogs. Using CRISPR-mediated knock-in approaches, I am reintroducing gene surrogates into zebrafish Hox clusters at the former locations of Hox14 paralogs. Using this “FauxHox” approach, we have introduced reporter constructs into the 14th position in zebrafish HoxAb and HoxDa clusters. These FauxA14 and FauxD14 lines exhibit nested, colinear FauxHox expression of their reporter gene product in the exact expression domains observed in species that retain Hox14 paralogs. This finding reveals that zebrafish Hox clusters retain the ability to regulate a 14th Hox cluster member despite losing Hox14 paralogs hundreds of millions of years ago. I now seek to reintroduce Hox14 coding regions into the zebrafish Hox clusters. Paired with studies of endogenous Hox14 paralogs in the non- model species that retain them, this analysis can reveal the role of Hox14 in forming the body plan, regulating the Hox cluster, and controlling the expression of target genes.